Home » Training » Medical Device Risk Management Training Courses » Medical Device Risk Management 3-Day/5 Half-Days
Medical Device Risk Management 3-Day or 5 Half-Days
- 3-Day or 5 half-days
- Corporate delivery (In-person or online)
- Certificate upon completion
- Professional Development Credits
Get Started Today
Register your interest now.
Let's Talk
Whether you have a question or are looking to find out more about our training options then please get in touch with us below.
- Summary
- Course Overview
- Course Outline
- Video
 Medical Devices Risk Management (MDRM) is a progressively more prominent topic in the medical device industry. Expectations by Regulatory bodies of medical device companies in producing quality, logical and defensible risk management files is rising. Manufacturers must convincingly demonstrate that the benefits of their medical devices outweigh the risks.
Medical Devices Risk Management (MDRM) is a progressively more prominent topic in the medical device industry. Expectations by Regulatory bodies of medical device companies in producing quality, logical and defensible risk management files is rising. Manufacturers must convincingly demonstrate that the benefits of their medical devices outweigh the risks.
This course provides a disciplined, systematic approach to analyzing, estimating, evaluating, and controlling safety risks related to medical devices. The methodology is explainable, logical, and integrated, utilizing formal risk management techniques to predict and prevent serious harm to patients and financial losses to businesses.
Participants will receive a comprehensive introduction to the essential topics for successfully managing safety risks of medical devices in conformance with the international standard ISO 14971.
Let’s Talk
Whether you have a question or are looking to find out more about our training options then please get in touch with us below.
The course is designed in multiple sections covering the critical topics that are necessary for risk management per ISO 14971. There are lectures on theory, multiple quizzes, and hands-on workshops to practice the techniques of medical device risk management, such as fault tree analysis and failure modes and effects analysis. Special topics such as medical device software risk management, and cybersecurity are also addressed in this course.
This course is the equivalent of a university graduate course taken by doctoral students in engineering.
Key Learning Objectives
At the conclusion of this course, participants are expected to have developed a sound working knowledge of medical device risk management in compliance with ISO 14971.
Training Methods and Materials
Participants are provided with a copy of the presentation materials. templates, and reference resources.
The companion textbook, “Safety Risk Management for Medical Devices” authored by the course facilitator is recommended for course participants. The book provides a substantial amount of additional materials, details, and step-by-step instructions on how to perform many of the presented risk management techniques. The book may be purchased from all major booksellers, e.g., Amazon, or directly from the publisher.
Please note: it is the course participant’s responsibility to purchase the book prior to course participation.
Who Should Attend this Course?
- Practitioners of medical device risk management – risk managers, systems engineers, design engineers, manufacturing engineers, quality engineers, usability engineers
- Evaluators of medical device risk management – quality assurance, management, regulatory staff
- People with a need for general understanding of medical device risk management – clinical, marketing, packaging, toxicology, management
Participants will not need prior knowledge or experience with medical device risk management, but experience in medical device development would be advantageous.
Do you Offer Tailoring of this Course?
Yes. A certain amount of tailoring is done to emphasize parts of the course that are of more interest to your team. We can also work with you to create customized training that is optimally suited to the needs of your company.
Course Availability
This course is available in a 3-day in-person format. Online delivery is tailored for 5 half-day sessions. Delivery dates will be coordinated to best suit your needs.
The Value Proposition for World Class Medical Device Risk Management
Proper risk management is a value-adding activity to medical device product development. Efficient, intelligent, and effective risk management ensures smooth product approvals, reduces field corrective actions, and achieves significant cost savings to the business.
Course Syllabus
1. Introduction to Medical Device Risk Management
- Why do we need to do medical device risk management?
- The benefits of medical device risk management
- History and origins of risk management
- Safety constraints
- Language of risk management
- Hazard theory
- Hazard Taxonomy
- How to identify reasonably foreseeable misuses
2. Medical Device Risk Management Standards
- ISO 14971 – the central standard in medical device risk management
- Risk management system
- Risk management process
- Requirements of ISO 14971
- Connections of ISO 14971 to: IEC 60601-1, IEC 62366, IEC 62304, ISO 10993, ISO 14155
3. Medical Device Risk Management as a Value-Added Activity
- How to use risk management to add value to product development
4. Medical Device Risk Management Activities and Artifacts
- Risk management plan
- Risk management report
- Risk management file
- Risk analysis, evaluation, control and monitoring
5. Foundations for Medical Device Risk Management
- Clinical Hazards List (CHL)
- What it is
- How to create it
- Harms Assessment List (HAL)
- What it is
- How to create it (two methods)
6. Medical Device Risk Management Tools and Techniques
- Fault Tree Analysis (FTA)
- Introduction
- FTA workflow
- Example FTA
- Failure Modes and Effects Analysis (FMEA/FMECA)
- Introduction
- Distinction between risk management and FMEA
- Relationship between FMEA and FTA
- How to leverage FMEAs to determine Essential Design Outputs (EDOs)
- Domains of Severity, Occurrence and Detectability
- Risk management scaling via hierarchical-multi-level FMEA
- DFMEA
- PFMEA
- Usability Engineering and risk management
- Use/Misuse FMEA (UMFMEA)
- Use failure distinctions
- UMFMEA
7. Software Risk Management
- Introduction
- Software failure model
- Language of SW risk management
- Contribution of software to system hazards
- Software risk
- Examples of SW faults
- Software FMEA
8. Cybersecurity and safety risk management
- Introduction to cybersecurity management
- Connection between cybersecurity and safety risk management
- Strategies for improving safety via cybersecurity
9. Medical Device Risk Assessment
- Preliminary Hazard Analysis (PHA)
- Risk Assessment and Control Table (RACT)
- Risk integration (FMEA, FTA, CHL, HAL, Risk Controls, Risk estimation & evaluation)
- RACT workflow
- P1 and P2, in-depth review
- Risk controls, distinction and proper crafting
- Safe by design/manufacture
- Protective Measures
- Information for safety
- Risk controls end-point logic (when to stop risk reduction)
- Verification of risk controls
- Residual risk estimation
- Boolean algebra and quantitative risk computation
- Traceability analysis
10. Benefit-Risk Analysis (BRA)
- FDA guidance
- Criteria for benefit-risk analysis
- BRA decision-making factors
11. Risk Management Review
- Requirements for risk management review
- Outcomes from risk management review
12. Post-market Medical Device Risk Management
- Basis and intention for post-market risk management
- Elements of post-market risk management
- Listening systems
- Surveillance
- Data monitoring
- Complaint handling
- Connection between post-market and premarket risk management
13. In Closing
- Common mistakes in medical device risk management
- Tips and wisdom for success
Best Practices in Risk Management
PPI Course Presenter and Principal Consultant Bijan Elahi, an expert on a world scale in medical device risk management, shares his wisdom in medical device risk management in this interview.
Bijan Elahi is also the author and presenter of PPI’s Medical Device Risk Management training course.
Featured Course Reviews

Risk Management for Safety-Critical Systems is an excellent practical course on risk management with a focus on medical technology. In addition to learning the fundamentals of risk management, in this course I learned a complete methodology for managing risk during the full product life cycle.
Participant, Netherlands
Philips

I am a candidate for the professional doctorate degree in Health Systems Design (PDEng) and took Bijan Elahi’s course on Risk Management for Medical Devices. During this very comprehensive course, Bijan not only gave us extensive insights to the Risk Management process (ISO14971), but also shared a great deal of useful and practical experiences in the industry.
Participant, Netherlands
Philips

This thorough and complete training unlocked many answers to questions I had when I was doing product development in the medical device industry. For example, why perform risk management? How exactly should we perform risk management? What happens in real-life situations? What does FDA do during audits, for example?, etc. I really enjoyed Bijan’s lectures and presentation, especially the interactive quizzes in class. They helped me reflect and absorb what I was learning.
Participant, Netherlands
Philips

Bijan’s expertise in the subject matter is unsurpassed and he explained everything in an easy-to-understand manner. He was very patient, inspiring, organized, passionate and open to suggestions. I really enjoyed this course and gained much professional knowledge. This is the most useful course I have taken in the PDEng.
Participant, Netherlands
Philips

Having vast industrial experience, Bijan also was able to use real life examples to easily relate the importance of risk management to product development and the do’s and don’ts. This course has given me the knowledge and expertise to practise risk management of medical devices in my own projects. This is one of the best and most relevant industrial courses that I have ever taken.
Participant, Netherlands
Philips
Thank you for the two days of FMEA and Risk training! The course material was excellent, the class style was engaging, and you are an insightful and great teacher. Our team, business, and devices will all benefit from this valuable experience.
Participant, USA
Medtronic
Great recap/lessons learned throughout the days of training
Participant, Denmark

Bijan is very knowledgeable and inspiring, open-minded
Participant, Denmark

Many examples were brought into the training
Participant, Denmark

Bijan was open to discussing and interpreting topics in different ways
Participant, Denmark
More Courses For You
Architectural
Design
Establish & maintain strong system solution relationships
CTI SE-ZERT®

Achieve SE-ZERT Certification
INCOSE SEP Exam Preparation Course 5-Day

Achieve INCOSE SEP Certification
Interface Engineering and Management 2 Days or 4 Half-Days
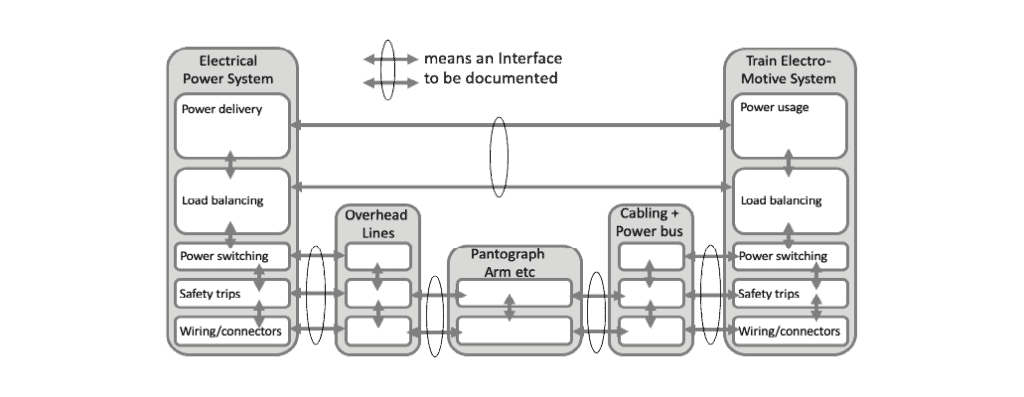
Avoid interface problems
Model-Based Systems Engineering (MBSE) Foundations 2-Day
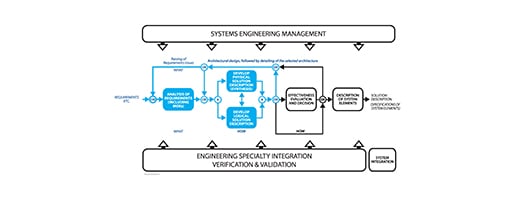
Organize your information in standard models for power & reuse
Systems
Engineering
Fulfilling the promise of innovation on demand
Systems Engineering Executive Overview Half-Day
ROI of SE practices
Systems Engineering Management 5-Day
Achieve SE & Program/Project Management alignment
Systems Engineering Overview 3-Day
Develop system solutions
