Bijan Elahi, Corporate Risk Management Consultant, Educator, and Technical Fellow, spoke about software safety at the Software Safety International Conference in Seoul.
Internationally recognized as a risk management expert and educator, Bijan Elahi, Medtronic Corporate Risk Management Consultant and PPI Course Presenter & Principal Consultant, recently spoke on “Software Safety: Lessons from the Medical Device Industry” at the 2019 Software Safety International Conference in Seoul, South Korea. The conference, hosted by the Korean Ministry of Science and Information and Communication Technologies (ICT), attracted about 200 attendees from academia, government, and industry.

“Many people don’t understand what safety really means,” he said, “but thanks to ISO 14971, those of us in the medical device industry have a clear definition of it as “freedom from unacceptable risk.”
In Bijan’s presentation he said, “In my keynote speech, I endeavored to bring to the audience clarity of knowledge about safety, risk, hazards, and harm,” adding that Software Risk Management cannot be addressed in isolation and must be analyzed within the system context. Software safety requires a multidisciplinary approach that includes input from systems engineering as well as software, mechanical, electrical, and usability engineering.
“Software itself is not a hazard,” says Bijan, “but it can contribute to hazards in the context of the system.” He shared several causes of software failure with his audience, including systemic defects, random software faults, and random hardware faults that could be caused, for example, by cosmic rays and air showers affecting modern electronics.
“Some people are dubious about cosmic rays,” he says, “but they are real.” In 2003 in Belgium, people started voting electronically in their elections. In one case, someone noticed that one of the contestants, a relatively unknown candidate, had received more votes from one polling station than there were voters! At first, they thought it was a case of fraud or tampering, so they recounted all the votes. This time, the candidate had 4,096 fewer votes. To software people, this is telling that 4096 = 2^12. The investigation showed there was no fraud or software error however, in contrary the computer memory tested fine. The only explanation for this false positive was the 13th bit in the integer that was the count of the candidate’s vote, had flipped from 0 to 1.
At the conference, Bijan presented certain techniques and strategies to help mitigate software risks through inspection, analysis, testing, defensive coding, and run-time error traps. The conference used a software app where the audience could ask questions to the speakers, enabling audience members to be more interactive. Thanks to the great reception of his speech, Bijan has been invited back to present at this years conference.
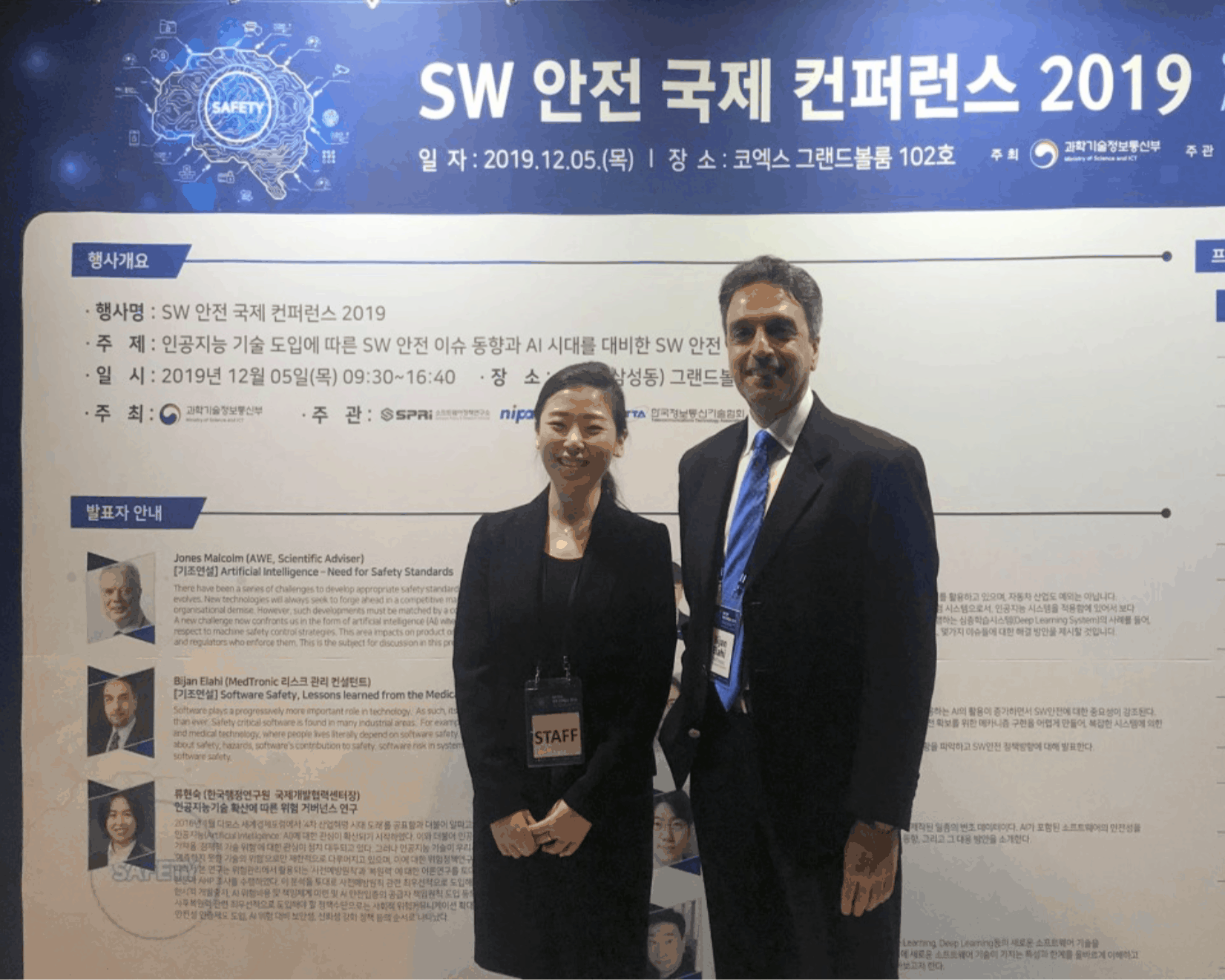
Yerin Seo, with Bijan, helped to coordinate Bijan’s visit.
Bijan has won several awards for his risk management expertise, including two from the International System Safety Society. In 2017, he won the society’s Educator of the Year Award in recognition of his outstanding performance in System Safety Education. In 2019, he won the society’s Scientific Research and Development Award in recognition of outstanding development of analytical methods to support Medical Device System Safety. He also is the recipient of the Medtronic Knowledge Management award of distinction for individual knowledge practices in 2019.
As Bijan teaches quantitative risk management, he says. “It is easier to explain, more objective, and conducive to computer automation. I believe in the future there will be a trend in the industry toward quantitative methods. Parts of Medtronic already use quantitative methods.”
Bijan is the author of the best-selling risk management book, “Safety Risk Management for Medical Devices.” Learn more about his book or order a copy by clicking here.
When asked what he is most proud of, Bijan said, “With the success of my book, my work at Medtronic, and my educational outreach outside of Medtronic, I feel I am really making a difference in the world and helping to make medical technology safer.”
For more information about Bijan and his background, visit Technical Fellow Bijan Elahi: Passionate about Safety and Risk Management.