New regulations on artificial intelligence have been proposed by the European Union to protect important public interests from what is deemed as high-risk systems. This includes medical devices and in vitro diagnostics. According to the EU’s plan, high-risk AI systems must comply with all of its mandatory requirements in order to be placed on the European market, and non-compliance will be penalised with fines that could total billions of dollars.
For some industries, the proposal may overlap with existing regulations. The EU has cited medical devices as an area in which this may occur due to the existing Medical Device Regulation. Yiannos Tolias, a lawyer at the European Commission’s health group, stated that the new horizontal AI proposal would complement existing regulations and handle AI specific concerns which may not be covered by MDR. According to Tolias, combining the two sets of regulations would make an AI medical device more secure and trustworthy.
Major penalties would be faced by companies that fail to comply with EU’s new regulations on AI. The EU plans to issue fines up to €30 million ($36 millionUSD), or, for companies that violate the rules, 6% of that company’s total worldwide annual turnover. This would total to almost $5 billion for all of Johnson & Johnson, and $1.7 billion for Medtronic based on their respective 2020 turnovers. In both cases, these fines would severely reduce profits.
The research community has raised concerns over the EU’s new proposal. Bob Carpenter, senior research scientist at the Flatiron Institute’s Center for Computational Mathematics, critiqued the proposal for being too vague in its definition of AI with phrasing such as “wide variety of methods” and “statistical approaches”. This criticism was echoed by other circles in the past for creating vague and complex regulations that bring unnecessary burden for its purpose. MDR has been described as “bureaucratic overreach”, and REACH has been slammed for compromising the competitiveness of the pharmaceutical industry.
The impact of the proposed regulations is not just limited to EU countries, in fact some laws may be adopted overseas to become the new standard. According to Forbes, changes in General Data Protection Regulation in 2018 similarly saw US companies spending $7.8 billion to adopt the changes.
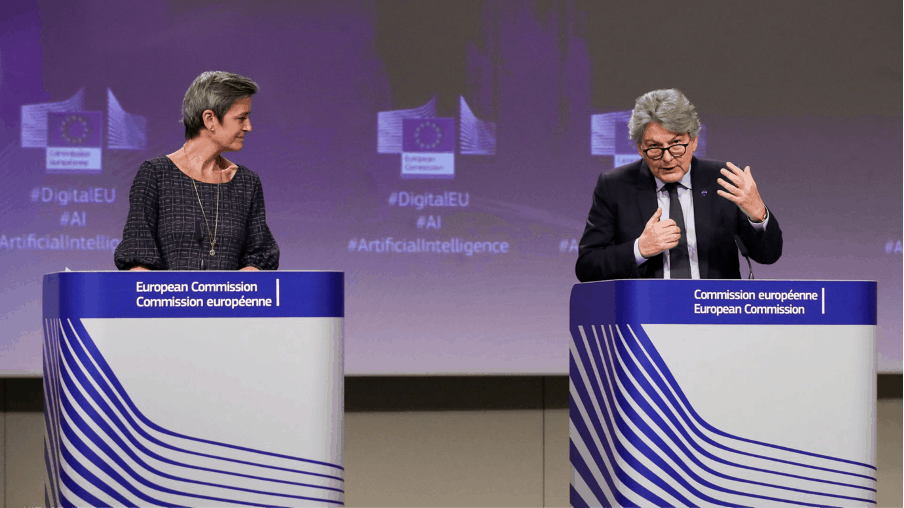
European Executive Vice-President Margrethe Vestager and European Internal Market Commissioner Thierry Breton give a media conference on the EU approach to Artificial Intelligence following weekly meeting of EU Commission in Brussels, Belgium, April 21, 2021. Olivier Hoslet/Pool via REUTERS
The trade-off for harsher regulations on AI is that they can potentially hinder its advancement. The current data infrastructure is seen as geographically fragmented. According to EIT Health, free exchange of data in Europe is necessary for creating (1) a more robust data infrastructure, (2) a larger database for experts to track and diagnose diseases, (3) new and improved AI-based solutions. A 2020 report by trade group MedTech Europe and Deloitte have shown similar findings, identifying the fragmented data landscape and different legal framework across regions as barriers to leveraging the full benefits of AI.
With an aging population, Europe could suffer more than other regions from lack of progress in AI in healthcare. The potential benefits of AI-based solutions can make healthcare more efficient. According to MedTech Europe, clearing the regulative barriers could save 400,000 lives a year and free up labour equal to the work time of 500,000 healthcare professionals.
References
Taylor, Nick Paul 2021, EU plans to impose additional regulations on medtech AI products, other ‘high-risk’ systems, 12th May 2021, https://www.medtechdive.com/news/eu-plans-to-impose-additional-regulations-on-medtech-ai-products-other-hi/600022/